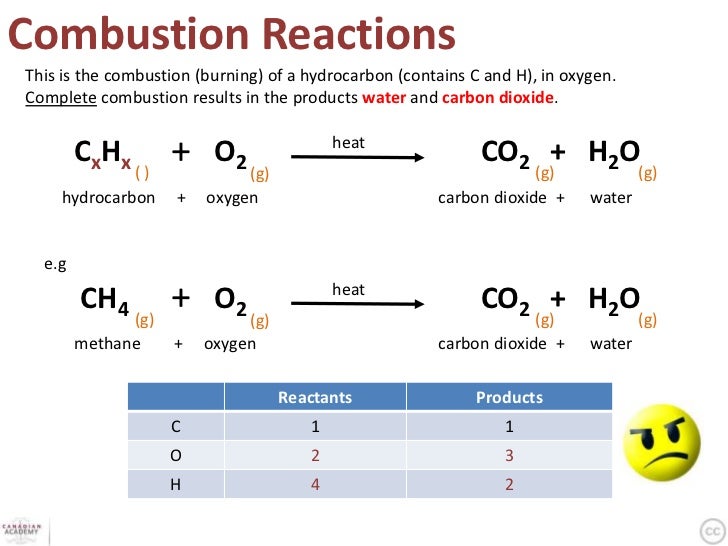
How To Test For The Products Of Complete Combustion . Here are the equations for the complete combustion of propane, used. Hydrocarbon + oxygen → carbon dioxide + water. the balanced equation for the process is: When a compound burns it reacts with oxygen. combustion analysis is a standard method of determining a chemical formula of a substance that contains. Catalytic converters can remove unburned hydrocarbons and nitrous oxides from fuel. The carbon and hydrogen atoms react with oxygen in an. 2.5.9 describe the complete combustion of alkanes to produce carbon dioxide and water, including observations and tests to identify the products. complete combustion occurs when there is a plentiful supply of oxygen. complete combustion happens when there is enough oxygen available, producing carbon dioxide (co2) and water (h2o) incomplete. Because of this we can predict what the products of the.
from www.slideshare.net
Here are the equations for the complete combustion of propane, used. the balanced equation for the process is: 2.5.9 describe the complete combustion of alkanes to produce carbon dioxide and water, including observations and tests to identify the products. The carbon and hydrogen atoms react with oxygen in an. Catalytic converters can remove unburned hydrocarbons and nitrous oxides from fuel. When a compound burns it reacts with oxygen. combustion analysis is a standard method of determining a chemical formula of a substance that contains. complete combustion happens when there is enough oxygen available, producing carbon dioxide (co2) and water (h2o) incomplete. Hydrocarbon + oxygen → carbon dioxide + water. complete combustion occurs when there is a plentiful supply of oxygen.
Reactions & Formulas
How To Test For The Products Of Complete Combustion combustion analysis is a standard method of determining a chemical formula of a substance that contains. Catalytic converters can remove unburned hydrocarbons and nitrous oxides from fuel. When a compound burns it reacts with oxygen. The carbon and hydrogen atoms react with oxygen in an. complete combustion occurs when there is a plentiful supply of oxygen. complete combustion happens when there is enough oxygen available, producing carbon dioxide (co2) and water (h2o) incomplete. 2.5.9 describe the complete combustion of alkanes to produce carbon dioxide and water, including observations and tests to identify the products. combustion analysis is a standard method of determining a chemical formula of a substance that contains. Because of this we can predict what the products of the. Hydrocarbon + oxygen → carbon dioxide + water. Here are the equations for the complete combustion of propane, used. the balanced equation for the process is:
From www.youtube.com
Complete & Combustion GCSE science, Chemistry (91) YouTube How To Test For The Products Of Complete Combustion complete combustion happens when there is enough oxygen available, producing carbon dioxide (co2) and water (h2o) incomplete. When a compound burns it reacts with oxygen. Hydrocarbon + oxygen → carbon dioxide + water. The carbon and hydrogen atoms react with oxygen in an. Catalytic converters can remove unburned hydrocarbons and nitrous oxides from fuel. Here are the equations for. How To Test For The Products Of Complete Combustion.
From www.youtube.com
∆Hrxn° combustion of methanol YouTube How To Test For The Products Of Complete Combustion Here are the equations for the complete combustion of propane, used. Catalytic converters can remove unburned hydrocarbons and nitrous oxides from fuel. complete combustion occurs when there is a plentiful supply of oxygen. Hydrocarbon + oxygen → carbon dioxide + water. When a compound burns it reacts with oxygen. 2.5.9 describe the complete combustion of alkanes to produce. How To Test For The Products Of Complete Combustion.
From www.wou.edu
CH150 Chapter 5 Chemical Reactions Chemistry How To Test For The Products Of Complete Combustion Because of this we can predict what the products of the. When a compound burns it reacts with oxygen. 2.5.9 describe the complete combustion of alkanes to produce carbon dioxide and water, including observations and tests to identify the products. Here are the equations for the complete combustion of propane, used. Hydrocarbon + oxygen → carbon dioxide + water.. How To Test For The Products Of Complete Combustion.
From invenoeng.com
No. 35 Combustion Testing Sheets How To Test For The Products Of Complete Combustion Hydrocarbon + oxygen → carbon dioxide + water. When a compound burns it reacts with oxygen. complete combustion happens when there is enough oxygen available, producing carbon dioxide (co2) and water (h2o) incomplete. the balanced equation for the process is: The carbon and hydrogen atoms react with oxygen in an. Because of this we can predict what the. How To Test For The Products Of Complete Combustion.
From www.tes.com
KS3 Science / Chemistry Combustion worksheet Teaching Resources How To Test For The Products Of Complete Combustion Because of this we can predict what the products of the. When a compound burns it reacts with oxygen. Catalytic converters can remove unburned hydrocarbons and nitrous oxides from fuel. complete combustion occurs when there is a plentiful supply of oxygen. Hydrocarbon + oxygen → carbon dioxide + water. combustion analysis is a standard method of determining a. How To Test For The Products Of Complete Combustion.
From www.teachoo.com
Case Based MCQ Chemistry in Automobiles For an internal combustion How To Test For The Products Of Complete Combustion Because of this we can predict what the products of the. Catalytic converters can remove unburned hydrocarbons and nitrous oxides from fuel. Hydrocarbon + oxygen → carbon dioxide + water. complete combustion occurs when there is a plentiful supply of oxygen. complete combustion happens when there is enough oxygen available, producing carbon dioxide (co2) and water (h2o) incomplete.. How To Test For The Products Of Complete Combustion.
From www.slideshare.net
Reactions & Formulas How To Test For The Products Of Complete Combustion Hydrocarbon + oxygen → carbon dioxide + water. The carbon and hydrogen atoms react with oxygen in an. Here are the equations for the complete combustion of propane, used. When a compound burns it reacts with oxygen. 2.5.9 describe the complete combustion of alkanes to produce carbon dioxide and water, including observations and tests to identify the products. Catalytic. How To Test For The Products Of Complete Combustion.
From www.youtube.com
TEST COMBUSTION YouTube How To Test For The Products Of Complete Combustion When a compound burns it reacts with oxygen. combustion analysis is a standard method of determining a chemical formula of a substance that contains. Because of this we can predict what the products of the. Catalytic converters can remove unburned hydrocarbons and nitrous oxides from fuel. Here are the equations for the complete combustion of propane, used. complete. How To Test For The Products Of Complete Combustion.
From accutools.com
Combustion Quick Start Guide AccuTools How To Test For The Products Of Complete Combustion The carbon and hydrogen atoms react with oxygen in an. combustion analysis is a standard method of determining a chemical formula of a substance that contains. Here are the equations for the complete combustion of propane, used. the balanced equation for the process is: 2.5.9 describe the complete combustion of alkanes to produce carbon dioxide and water,. How To Test For The Products Of Complete Combustion.
From www.youtube.com
Material Balances on Complete Combustion of Methane YouTube How To Test For The Products Of Complete Combustion 2.5.9 describe the complete combustion of alkanes to produce carbon dioxide and water, including observations and tests to identify the products. Here are the equations for the complete combustion of propane, used. When a compound burns it reacts with oxygen. Hydrocarbon + oxygen → carbon dioxide + water. combustion analysis is a standard method of determining a chemical. How To Test For The Products Of Complete Combustion.
From www.youtube.com
GCSE Chemistry 19 Combustion How is it different to How To Test For The Products Of Complete Combustion Hydrocarbon + oxygen → carbon dioxide + water. complete combustion happens when there is enough oxygen available, producing carbon dioxide (co2) and water (h2o) incomplete. the balanced equation for the process is: Catalytic converters can remove unburned hydrocarbons and nitrous oxides from fuel. 2.5.9 describe the complete combustion of alkanes to produce carbon dioxide and water, including. How To Test For The Products Of Complete Combustion.
From www.slideserve.com
PPT Combustion PowerPoint Presentation, free download ID5525141 How To Test For The Products Of Complete Combustion 2.5.9 describe the complete combustion of alkanes to produce carbon dioxide and water, including observations and tests to identify the products. combustion analysis is a standard method of determining a chemical formula of a substance that contains. The carbon and hydrogen atoms react with oxygen in an. the balanced equation for the process is: When a compound. How To Test For The Products Of Complete Combustion.
From www.sciencephoto.com
Complete combustion experiment Stock Image C025/6910 Science How To Test For The Products Of Complete Combustion When a compound burns it reacts with oxygen. complete combustion occurs when there is a plentiful supply of oxygen. The carbon and hydrogen atoms react with oxygen in an. Because of this we can predict what the products of the. 2.5.9 describe the complete combustion of alkanes to produce carbon dioxide and water, including observations and tests to. How To Test For The Products Of Complete Combustion.
From www.youtube.com
Combustion hexane with dry molar analysis YouTube How To Test For The Products Of Complete Combustion The carbon and hydrogen atoms react with oxygen in an. the balanced equation for the process is: complete combustion occurs when there is a plentiful supply of oxygen. Hydrocarbon + oxygen → carbon dioxide + water. When a compound burns it reacts with oxygen. complete combustion happens when there is enough oxygen available, producing carbon dioxide (co2). How To Test For The Products Of Complete Combustion.
From wendy-kkey.blogspot.com
Which of the Following Combustion Reactions Is Balanced Correctly How To Test For The Products Of Complete Combustion Because of this we can predict what the products of the. the balanced equation for the process is: combustion analysis is a standard method of determining a chemical formula of a substance that contains. complete combustion occurs when there is a plentiful supply of oxygen. Catalytic converters can remove unburned hydrocarbons and nitrous oxides from fuel. Hydrocarbon. How To Test For The Products Of Complete Combustion.
From www.slideserve.com
PPT MODULE 5 PowerPoint Presentation, free download ID419030 How To Test For The Products Of Complete Combustion Catalytic converters can remove unburned hydrocarbons and nitrous oxides from fuel. Because of this we can predict what the products of the. Hydrocarbon + oxygen → carbon dioxide + water. complete combustion happens when there is enough oxygen available, producing carbon dioxide (co2) and water (h2o) incomplete. 2.5.9 describe the complete combustion of alkanes to produce carbon dioxide. How To Test For The Products Of Complete Combustion.
From www.youtube.com
Chemical Reactions (3 of 11) Combustion Reactions, An Explanation YouTube How To Test For The Products Of Complete Combustion Hydrocarbon + oxygen → carbon dioxide + water. Here are the equations for the complete combustion of propane, used. Catalytic converters can remove unburned hydrocarbons and nitrous oxides from fuel. complete combustion occurs when there is a plentiful supply of oxygen. 2.5.9 describe the complete combustion of alkanes to produce carbon dioxide and water, including observations and tests. How To Test For The Products Of Complete Combustion.
From www.shalom-education.com
Fuels and Combustion KS3 Chemistry Revision How To Test For The Products Of Complete Combustion complete combustion occurs when there is a plentiful supply of oxygen. Here are the equations for the complete combustion of propane, used. 2.5.9 describe the complete combustion of alkanes to produce carbon dioxide and water, including observations and tests to identify the products. Because of this we can predict what the products of the. Hydrocarbon + oxygen →. How To Test For The Products Of Complete Combustion.